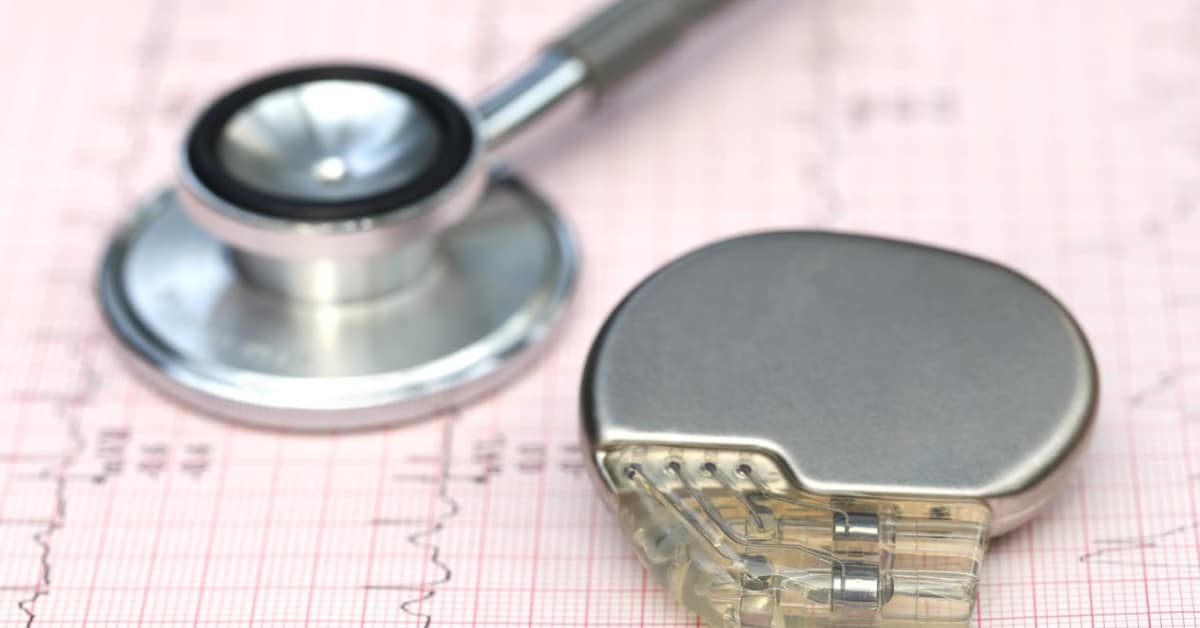
Earlier this year, the U.S Food and Drug Administration (FDA) issued two warning letters to Medtronic. During an inspection of Medtronic’s Cardiac Rhythm and Heart Failure (CRHF) business, from April 23 through May 14, 2018, the FDA found manufacturing nonconformities, which resulted in a Medtronic defibrillator recall. A Medtronic recall, earlier this year in January, occurred due to a defect in the manufacturing process, which the FDA identified as a Class I recall.
What the Medtronic Recalls Entail
The FDA inspected two Medtronic facilities locations, which included the Medtronic Cardiac Rhythm and Heart Failure (CRHF) site in Minnesota and the Medtronic Puerto Rico Operations Company (MPROC) site. While inspecting both sites, the FDA found manufacturing issues with two of the products. Those products included the Medtronic Cardiac Resynchronization Therapy with Defibrillation (CRT-Ds) and Implantable Cardiovert-Defibrillators (ICDs) models. A total of nine CRT-Ds and twelve ICD models were found to be defected and included in the recall. One of the issues the FDA emphasized in the letters was the failure to document specific activities in the implantable cardiac defibrillators’ device history records. The FDA also noted violations of current good manufacturing at the sites.
As a result of the letters, the Medtronic recalls were voluntary, with Medtronic also informing care providers to consider replacing the affected ICDs, including the Evera and Visia models, with prophylactic devices. The defects found in the Medtronic pacemaker and defibrillator models were discovered to cause an “out of specification gas mixture inside the device which may prohibit the device from delivering the electrical shock needed to pace a patient’s heartbeat or revive a patient in cardiac arrest,” as stated by the FDA. Such delay or inability to deliver a shock to a patient in cardiac arrest or pace a patient’s heart with a heartbeat that is too slow could result in serious injury and/or death. Therefore, the Medtronic pacemaker recall was labeled by the FDA as “the most serious type due to the identified defect having the ability to prevent the CRT-Ds and ICDS from delivering the life-saving, electrical shock therapies patients need.”
Medtronic Defibrillator Lawsuit
Although no one has succumbed to injury or death from the defected Medtronic products, all physicians have been notified to continuously follow the 48 patients who currently have affected devices. It is not entirely possible, however, to determine which of these 48 devices may fail or when they may fail. In addition to that, the number of medical device recalls has been increasing over the years, reaching a total of 3,202 last year and exceeding the 2,876 recalls in 2016.
Overall, a total of 526 Medtronic devices have been recalled, just in the United States. Although no deaths or injuries have been reported as of yet, the defected Medtronic devices pose many risks for individuals and can result in severe consequences.
If you or a loved one have been the victim of a defective Medtronic recall device, please contact the Eichholz Law Firm. We can assist in advising you with your Medtronic defibrillator lawsuit and will get you the compensation you deserve.